close
Choose Your Site
Global
Social Media
| Availability: | |
|---|---|
| Quantity: | |

98% magnolol、Honokiol
World-way

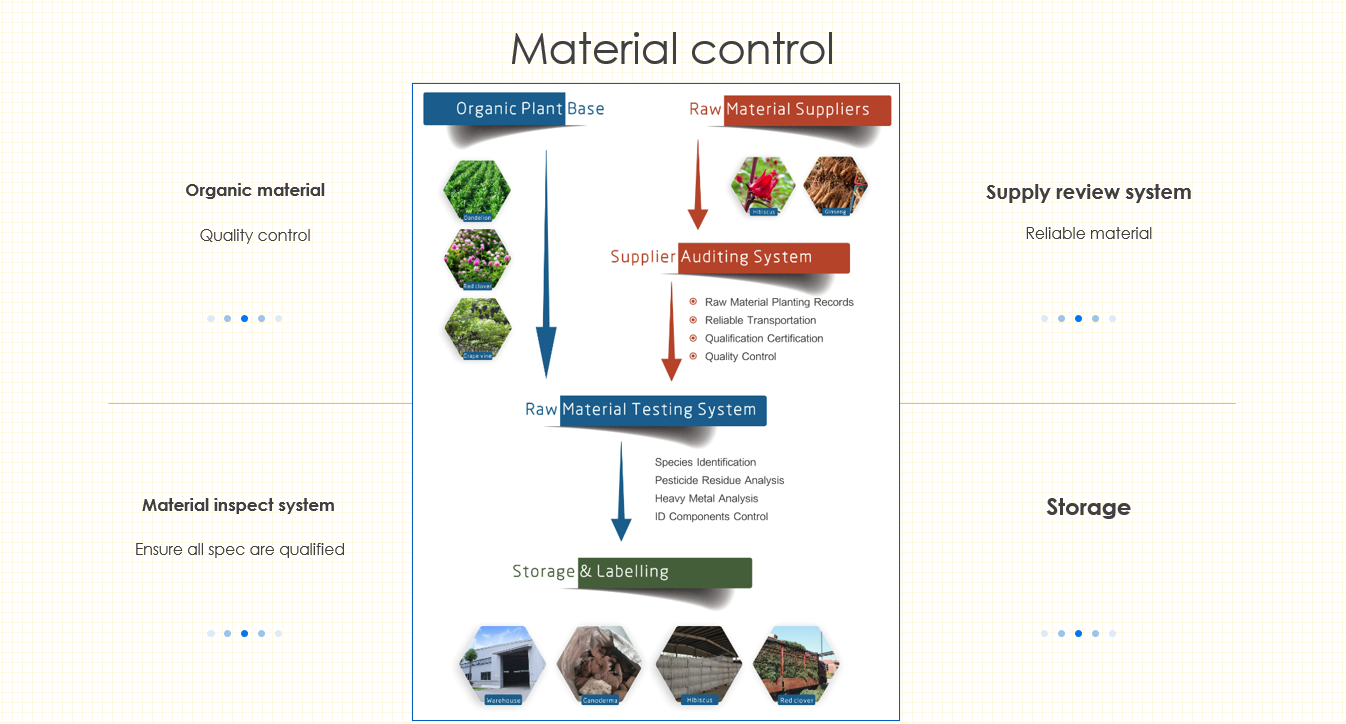
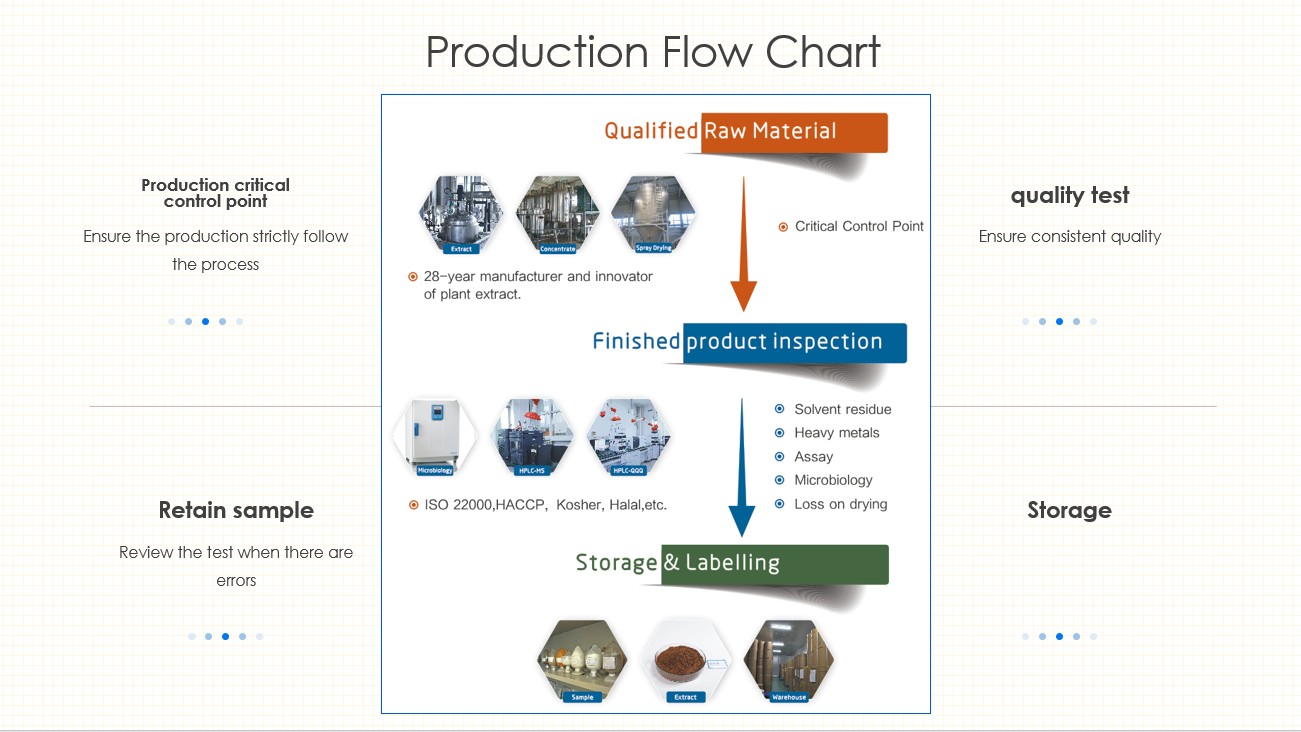
Magnolia(magnolia officinalis) is native to China, is natural compound used in traditional Chinese practices and known for its ability to support healthy sleep habits and its relaxation properties. The primary primary active ingredients -Honokiol and Magnolol are CO2 extracted from magnolia bark which can be used for dietary supplements, toothpaste, and topical oils and cream.


1.Anti-bacteria,treat halitosis and prevent teeth decay;
2.Anti-cancer,anti-oxidant;
3.Anti-virus,anti-ulcer and anti-inflammatory;
4.Relieve anxiety and stress,inhibits cortisol,anti-depression,treat sleeping problems.


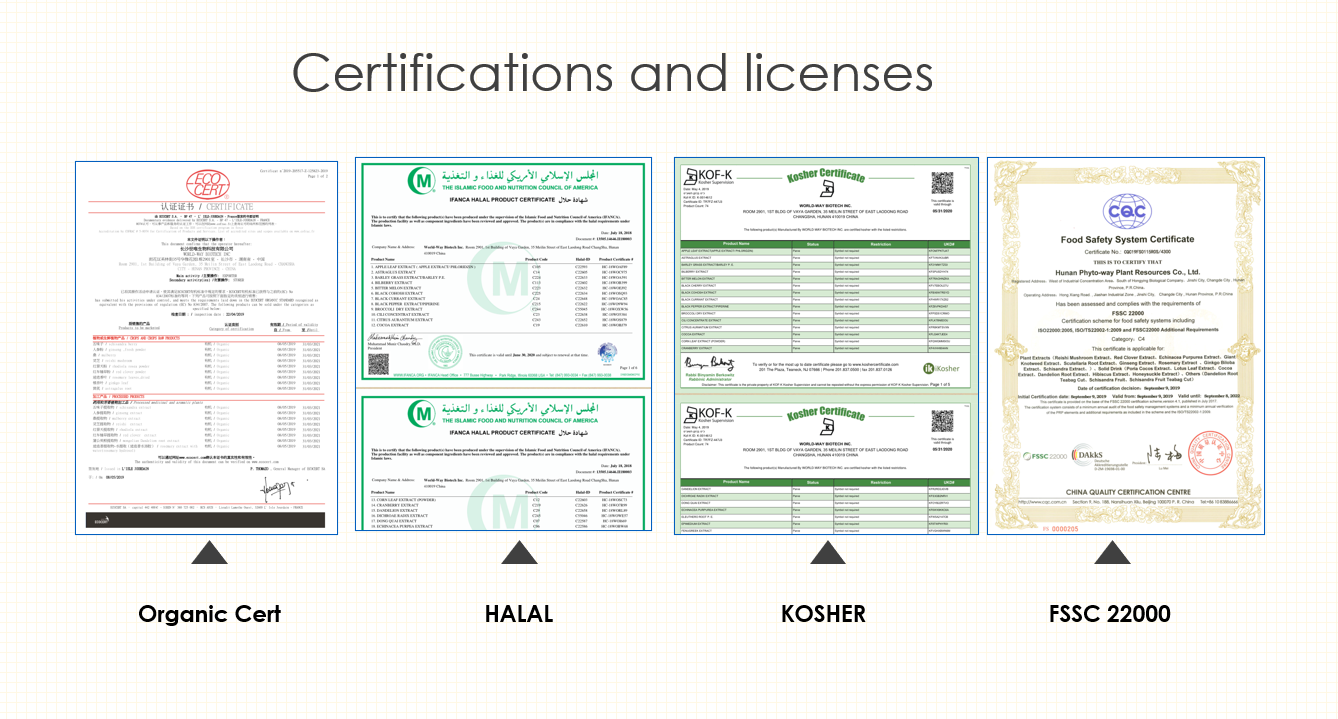
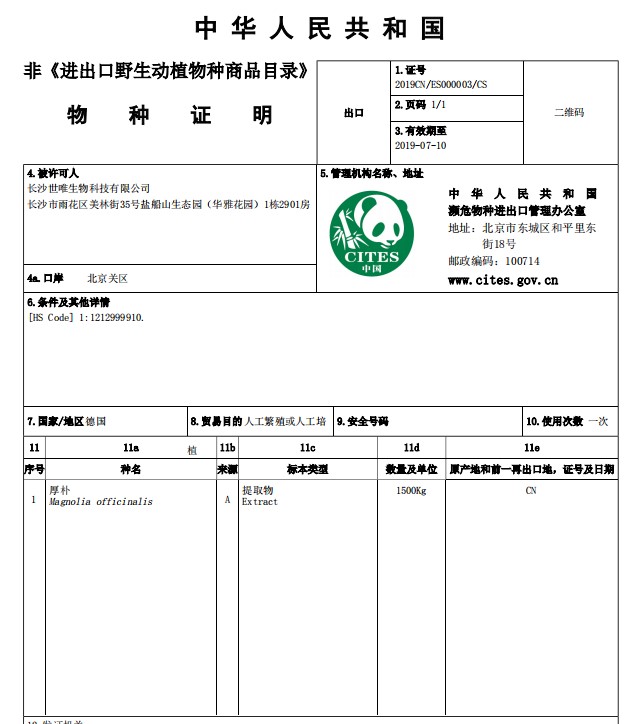
NON-CITES for Magnolia Bark Extract

Magnolia(magnolia officinalis) is native to China, is natural compound used in traditional Chinese practices and known for its ability to support healthy sleep habits and its relaxation properties. The primary primary active ingredients -Honokiol and Magnolol are CO2 extracted from magnolia bark which can be used for dietary supplements, toothpaste, and topical oils and cream.


1.Anti-bacteria,treat halitosis and prevent teeth decay;
2.Anti-cancer,anti-oxidant;
3.Anti-virus,anti-ulcer and anti-inflammatory;
4.Relieve anxiety and stress,inhibits cortisol,anti-depression,treat sleeping problems.


NON-CITES for Magnolia Bark Extract